The Power of Adaptive Learning
Effective protocol training is essential to the success of any clinical trial, but can be challenging due to the complexity of protocols, presentation of novel procedures and varied staff skill and experience both within and across sites. If training is to ensure optimal performance by all research staff, it must address those challenges. Adaptive learning approaches are uniquely positioned to overcome all these challenges, while also reducing the time burden placed on research site staff by training and re-training.
Adaptive learning is a data-driven approach to instruction that allows material to be adjusted and tailored to meet the individual needs of different learners, rather than a one-size-fits-all experience. This approach uses computer software to create customized experiences and materials based individual learners’ skills and experience level. It provides feedback and resources unique to each person.
Adaptive learning is typically done using a basic “if A happens, then B happens” approach, a June 2022 article on the Leader.com website explains. For instance, if a learner gives a wrong answer, the system would offer guidance and prompt them to retry; conversely, individuals may skim through areas where they already have mastered the material.
Originally introduced in education, adaptive learning systems have been making inroads into many types of business training systems, as well, including medical fields. In a 2017 blog post on its website, for instance, the New England Journal of Medicine (NEJM) pointed to several common scenarios where adaptive learning is shown to outperform other approaches.
For instance, when learning outcomes—such as performance of a particular medical procedure—have serious consequences, adaptive learning has been shown to better ensure proficiency when the employee performs that procedure on actual patients. It also outperforms other types of training when the intended audience is heterogeneous, with a variety of experience levels and skill sets, a situation that is common in clinical research.
Adaptive learning is also especially useful when time is of the essence. Clinical research staff often complain of being overburdened with administrative tasks like training. An adaptive learning system, with its ability to focus on knowledge gaps and build proficiency faster, can minimize the amount of time busy researchers must spend on training, while still ensuring top performance.
Other scenarios where adaptive learning outperforms other types of training, according to the NEJM blog post, include when training must be repeated over time, which can result in both new and old information being presented to the same individuals repeatedly, and when information is changing frequently, which is always the case in evidence-based medicine and clinical research.
This ability to essentially skip known material or to “test out” of some training where they already are proficient could be a particular boon in the clinical research arena. The time needed for training, particularly for re-training on the same material, is a frequent complaint, as indicated by a 2020 HealthStream survey on adaptive learning. This approach ensures that more skilled and experienced learners focus strictly on novel instruction.
Pick the right platform for adaptability
Taking advantage of the pluses offered by adaptive learning requires the right training platform. For instance, Pro-ficiency’s simulation-based training platform allows for adaptive learning in several ways. The system’s proprietary content authoring tool enables rapid construction and maintenance of branching logic, “choose your own adventure” learning modules (see Figure 1 below).
Figure 1: Branching Logic in Pro-ficiency’s Platform
In this way, all learners can take slightly different paths through the learning module, depending on their baseline capabilities and knowledge. The system will train individuals only when they make mistakes. When they make correct choices, they can quickly advance to the next challenge. This can reduce training time by up to 50%.
Further, each role on a study team has customized learning content in the system, based on job-specific target competencies. In this way, the platform adapts to the specific needs of each participant and team member of the study or internal sponsor team. This differs from video or slide training, where a one-hour training session will always take one hour, regardless of an individual learner’s baseline knowledge and skill sets.
The system also offers predictive analytics that enable detection of trends in performance data. This data can predict strengths and weaknesses in site staff, CRA and monitor competencies, allowing it to adapt smoothly to the needs of the entire cohort of learners.
Figure 2, below, provides an example of this capability. It shows performance results from three research sites that completed simulation-based training for a Phase 3 protocol. The vertical columns show the competencies while the horizontal rows represent the individuals who have completed the training. The trifurcation indicates the three separate sites.
A grey checkmark indicates that the individual passed that challenge with no need for mentoring or training. Figure 2 indicates that the first site did very well across the board, as did the third site, except for the CRA three rows from the bottom. This information lets study leadership know that the CRA at the third site requires additional attention and training. The middle site, on the other hand, is struggling except for one individual; study leaders can leverage the high performance of that person to act as a champion to mentor and guide other team members.
Figure 2: Predictive Analytics Generated During Pro-ficiency Training
This performance data can also highlight areas of a protocol that may be more challenging. Figure 2 shows such a situation, with one particular competency that everyone struggled with, high and low performers alike. That competency will require more targeted remediation, which the system will provide.
And the study team is not the only group that can benefit from this adaptive learning approach. Study participants can also experience similar training, via Pro-ficiency’s ProPatient product.
Because Pro-ficiency’s simulation-enabled performance management system is bespoke-built for clinical trials, it also offers capabilities to solve problems that most sponsors have not yet even identified. One of these is amendment management. Every protocol amendment increases complexity in the study by creating a new cohort within the staff for employees trained on A1 versus A2 versus A3, and so on. High staff and CRA turnover further add to this complexity.
The standard response to this challenge is to force constant retraining on affected sites or internal teams as amendments may require. The Pro-ficiency system, however, tracks exactly which competencies and which version each learner has completed. This allows retraining to focus only on new material, which dramatically reduces training burden on research staff.
`The final point concerning adaptive learning relates to how the data generated from the training system helps study teams adapt their monitoring plans. For instance, data like that shown in Figure 3, below, informs the study team as to which sites are more likely to generate study errors. This allows monitoring resources to be more effectively targeted—or adapted—to the riskiest sites at the beginning of the study. Sponsors don’t have to wait for problems to occur; they can address weak areas proactively.
Figure 3: Predicted Error Rates Via Pro-ficiency Training
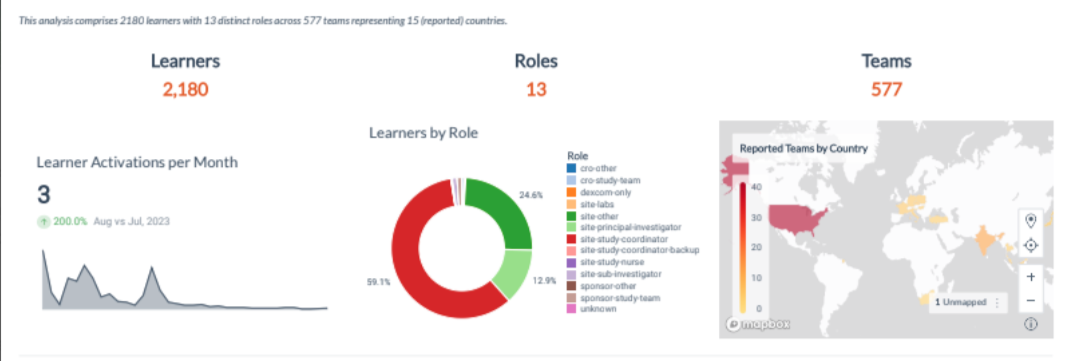
This also extends to global considerations where culture, language or other country-specific issues may impact research staff performance. Pro-ficiency provides a dashboard that displays the predictive performance analytics in real-time and spread geographically according to the distribution of sites and teams.
Training in the clinical research industry will continue to pose a variety of challenges, including time constraints, ever-changing information due to protocol amendments or new scientific evidence, novel procedures that fall outside research staff experience and a broad range of skill level both within and across research sites. Any training program lacking the agility to handle these challenges is doomed to failure, leading to mistakes and delays in getting investigational products to market.
Adaptive learning offers a powerful solution to those challenges. By adopting training platforms with solid adaptive learning features, such as that offered by Pro-ficiency, new product sponsors can help ensure a faster, smoother path through the clinical research process.
To learn more about Pro-ficiency’s simulation-based training solution, please visit our website: https://pro-ficiency.com/simulation-training

Dave Hadden is an entrepreneur and technology innovator, founding Pro-ficiency and pioneering the fields of A.I.-based medical decision-support, Training Analytics, and Virtual Patient Simulation (VPS). Dave has focused his passion for technological innovation and learning systems in the field of clinical trials, helping sponsors make their studies more accurate and efficient through finding the right technology mix such as virtualization, performance management and applied behavioral sciences to produce the most effective, lasting, and engaging results for clinical trials.







